What is burning?
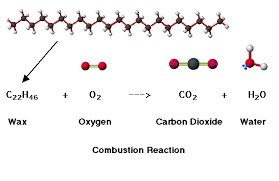
- Oxygen in what is burning up while a candle is on fire. It is the oxygen in the atmosphere that chemically combines with the wax of the candle to give Carbon dioxide gas and water vapor.
Write the chemical reaction?
When you burn a candle, you are performing simple combustion reactions of this type. The oxygen comes from the air and the heat initially comes from an outside source such as a match. When this combustion reaction happens, it makes water and carbon dioxide as shown as well as heat and light energy. The coefficients tell us how much of each component is used and produced.
The wick in a candle is also mainly hydrocarbon with oxygen also present and this also burnms though at a much slower rate. When you light the wick, you provide a heat source and oxygen from the air. The smoke formed contains carbon dioxide and water and also some soot from inneficient combustion.
wax formula- C25H52
Write the balanced chemical reaction.
Balanced: C25H52 + 38 O2 → 25 CO2 + 26 H2O
Butane- C4H10+ 1O2------ 8H2O+ 4CO2
balanced= 2c4h10+13o2= 10h2o+8co2

No comments:
Post a Comment